As part of our ongoing BBSRC-funded iCASE studentship with the University of Liverpool, we are exploring the use of CPNs™ for in vivo live cell imaging in regenerative medicine for stem cell tracking. The goal of the project is to use CPNs™ to better understand cell activity, which is crucial in the development of cell-based therapies.
Thanks to their powerful brightness, magnetic core and availability in a range of colours, CPNs™ can be deployed in a variety of key imaging techniques. In addition, their stability means they can be stored for long periods with no loss of brightness. By using dual labelling with a contrasting agent, CPNs™ can be used in bioluminescence imaging (BLI), fluorescence imaging (FI) and magnetic resonance imaging (MRI) to track single cells, both in two and three dimensions. The images produced with fluorescence can therefore aid our understanding of how cells interact with host tissue, how they differentiate and their survival and migratory capabilities.
BLI and MRI have been shown to be effective for non-invasive in vivo imaging of engineered tumour models. Using CPNs™ in these types of studies has the potential to improve in vivo imaging capability, providing further insight into cell activity, and ultimately informing the development of cell-based therapies.
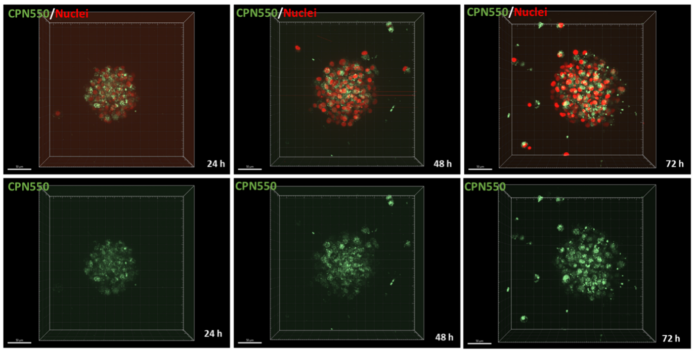
We have demonstrated that CPNs™ can be used to label cancer cells (U87 glioblastoma cells) in vitro for 7 days, without causing any toxicity to cells. In addition, CPNs™ can label cancer cells in 3D spheroids in vitro. This shows the potential of CPNs™ in enhancing our understanding of cancer biology.
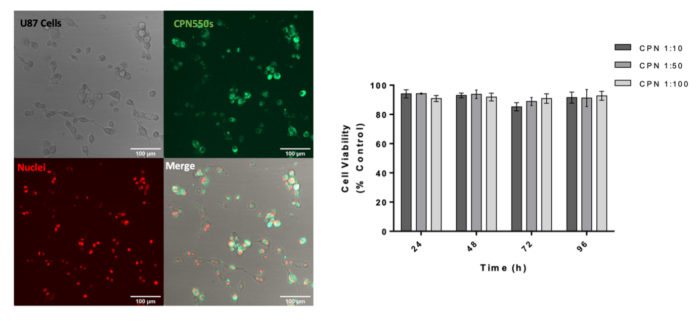
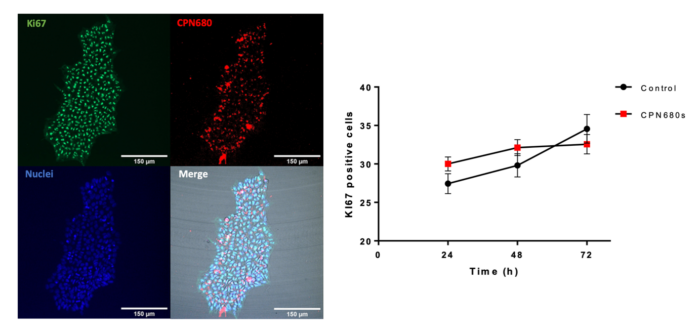
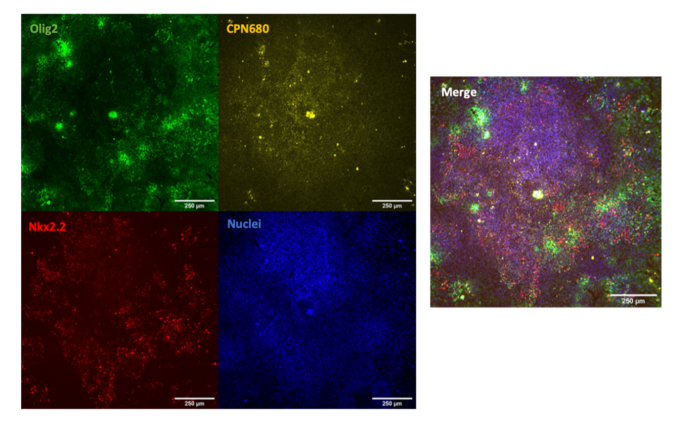
CPNs™ can also be used to label undifferentiated H9 human embryonic stem cells (hESCs) with no toxicity and no effect on the ability of the cells to proliferate or to differentiate towards oligodendrocyte precursor cells, as shown below. This potential application of CPNs™ in stem cell labelling has wider implications for studying regenerative therapies.
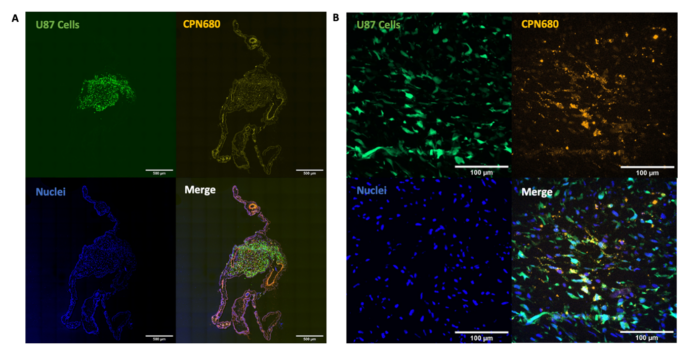
Finally, we have shown that CPNs™ can be used for cell labelling in in vivo models. This shows the potential of CPNs™ to enhance in vivo cell tracking studies in a range of models to further advance cancer research and regenerative medicine.